Contributed by C. Hiebert
Lr32 is a gene transferred from Aegilops tauschii to chromosome arm 3DS of hexaploid wheat that confers seedling stage resistance to leaf rust (1,2). The original accession of A. tauschii in which Lr32 was found was RL5497-1 (2n = 14 = DD), which was crossed with Tetra Canthatch (2n = 28 = AABB) to produce the synthetic hexaploid line RL5713. Lr32 was transferred from RL5713 to RL6086 (Thatcher*7//RL5713/MarquisK) and to BW196 (Katepwa*6//RL5713/2*MarquisK).
BW196 was registered in Canada but not released as a cultivar. A recent increase contained approximately 30% plants that were susceptible to leaf rust race MBDS (12-3) and some anomalous plants that were resistant but did not have markers derived from RL5497. The rest of the plants (approx. 60%) combined leaf rust resistance with 3DS-specific marker alleles that were introgressed from RL5497. Many of these alleles were also present in RL6086. However, since more RL5713 alleles were found in BW196, a single marker resistant reselection named K1160 (BW196R) was selected and crossed with a plant of Thatcher. The F1 was used to make a population of 361 doubled haploid (DH) lines. A selection of 338 lines from the DH population was used to create a provisional genetic map of the A. tauschii introgression into BW196.
Lr32 conferred resistance to 616 different isolates of Puccinia triticina obtained from wheat fields in Canada between 2000 and 2009. Singh (3) reported no case of virulence on Lr32 among 325 isolates collected in Mexico between 1988 and 1989 and no virulence has been noted in Australia (4). Pretorius and Bender (5) demonstrated virulence on Lr32 in a rare virulence phenotype isolated from triticale in the western Cape (South Africa).
Effect of Lr32 on Agronomics and Quality
Both Lr32-carrying lines, BW196 and RL6086, were tested on the field for effects of the leaf rust resistance gene on a series of agronomic and quality related traits. The introduction of Lr32 in Katepwa had no significant effect on the traits assessed, except for small reduction in plant height and test weight and an increase in kernel size. The effect of Lr32 in Thatcher was more marked, there were increases in yield, straw strength, test weight, kernel size and kernel hardness. Some of these changes could be pleiotropic effects caused by the later maturity of RL6086 compared to Thatcher.
Map Position of Lr32
Thomas et al. (6) found nine polymorphic markers between BW196R and Thatcher located on a linkage block on 3BS that included Lr32. The markers that mapped closest to Lr32 were Xwmc43 and Xbarc135, which cosegregated 0.6 cM proximal to Lr32. An unequal segregation ratio for Lr32 suggests a preference for the recovery of T. aestivum chromosome material against the A. tauschii segment. In RL6086 the segment introgressed from A. tauschii into Thatcher was shorter, and did not include the A. tauschii marker alleles expected on the distal side of Lr32.
Primers:
WMC43-F 5'- TAG CTC AAC CAC CAC CCT ACT G -3'
WMC43-R 5'- ACT TCA ACA TCC AAA CTG ACC G -3'
BARC135-F 5'- ATC GCC ATC TCC TCT ACC A -3'
BARC135-R 5'- GCG AAC CCA TGT GCT AAG T -3'
PCR conditions
- Denaturing step: 94°C, 2 min
- Amplification step (30 cycles):
- 95°C, 1 min (0.5°C/s to 61/51°C)
- 61°C (wmc43) / 51°C (barc135), 50 sec (0.5°C/s to 73°C)
- 73°C, 1 min
- Extension step: 73°C, 5 min
PCR reaction mix
Marker amplification was performed using M13 tailing for fluorescence separation which required adding the M13 sequence (CACGACGTTGTAAAACGAC) to the 5' end of the forward primer during primer synthesis.
- PCR buffer 1X
- 0.8 mM each dNTP
- 1.5 mM MgCl2
- 50 mM KCl
- 2 pmol reverse primer
- 0.2 pmol forward primer
- 1.8 pmol M13 primer (CACGACGTTGTAAAACGAC) fluorescently labelled with 6-FAM, VIC, NED, or PET
- 0.5 U Taq DNA polymerase
- 24 ng genomic DNA
Expected products and marker assisted selection
The separation and analysis of fluorescent fragments was done using capillary electrophoresis on an Applied Biosystems ABI 3100 genetic analyzer. Fragment sizes are reported without the 19-bp M13 tail.
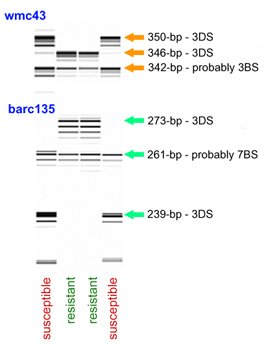
Marker Assisted Selection with wmc43
The Xwmc43 allele linked to Lr32 amplified a PCR product of 346 bp, while the allele of the recurrent parent Thatcher had a size of 350 bp. The 346-bp allele was absent in a survey of 493 common wheat lines lacking Lr32 from different origins. In these lines the most common variant (99.5%) was a pair of fragments, the larger ranged between 348 and 352 bp and the smaller between 336-344 bp. The other infrequent variants were observed in an Iranian landrace (KU4625) that had two small fragments of 338 and 342 bp, and in line BW674 that amplified only one fragment of 342 bp.
Marker Assisted Selection with barc135
Lines carrying Lr32 amplified a fragment of ~273 bp while the size of the Thatcher allele was ~239 bp. In a panel of 252 wheats of diverse origins the most frequent allele (98%) was a Thatcher-type fragment. A second larger and somewhat more polymorphic fragment was universally present in common wheat, typically migrating at ~261 bp.
References
1.Resistance to leaf rust in hexaploid wheat: Lr32, a third gene derived from Triticum tauschii. Kerber ER. In: Crop Science, 1987, 27:204-206 [Journal link].
2.Telocentric mapping in wheat of the gene Lr32 for resistance to leaf rust. Kerber ER. In: Crop Science, 1988, 28:178-179 [Journal link].
3. Pathogenicity variations of Puccinia recondita f. sp. tritici and P. graminis f. sp. tritici in wheat growing areas of Mexico during 1998 and 1999. Singh RP. In: Plant Pathology, 1991, 39:424-433.
4. Wheat rusts: An atlas of resistance genes. McIntosh RA, Wellings CR, Park RF. 1995. Kluwer Academic Publishers, Dordrecht, Boston and London.
5. First report of virulence for the wheat leaf rust (Puccinia triticina) resistance gene Lr32 in South Africa. Pretorius ZA, Bender CM. In: Plant Disease, 2010, 94:381. DOI:10.1094/PDIS-94-3-0381A
6. Genetic Markers and Leaf Rust Resistance of the Wheat Gene Lr32. Thomas J, Nilmalgoda S, Hiebert C, McCallum B, Humphreys G, DePauw R. In: Crop Science, 2010, 50:2310-2317. DOI:10.2135/cropsci2010.02.0065
