Stem rust resistance gene Sr25, leaf rust resistance gene Lr19, Semolina color gene Y
Contributed by Long-Xi Yu and Mark Sorrells. PSY-specific markers contributed by Jorge Dubcovsky
Markers for Sr25
Sr25 was transferred into wheat from Thinopyrum ponticum Barkworth and Dewey and is effective to Ug99. Sr25 and the linked leaf rust resistance gene Lr19 were translocated onto the long arm of wheat chromosomes 7D (1) and 7A (2). The use of germplasm containing Sr25/Lr19 was initially limited because of linkage with another Th. ponticum derived gene causing undesirable yellow flour. Knott (3) developed a mutant line, ‘Agatha-28’, containing Sr25/Lr19 with reduced yellow color due to a mutation in the PSY-E1 gene (4). It was then backcrossed into Australian wheat varieties and has been used in the CIMMYT wheat breeding program where it is present in the variety ‘Wheatear’ (5). Sr25 is distal to Lr19, and maps closely linked to PSY-E1 (J. Dubcovsky personal communication).
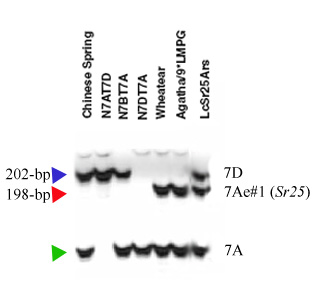
The co-dominant marker BF145935 derived from a wheat EST (6) and the dominant marker, Gb, were used for haplotyping Sr25(7). Liu et al. (8) validated marker BF145935 using 42 wheat lines and indicated that two DNA fragments amplified in most lines. The upper band of Wheatear was smaller than that of non-Sr25 lines. This DNA fragment is located on the 7Ae#1 segment that was translocated onto wheat chromosome 7DL (6, 8).
The introgression of the 7EL segment including Lr19 and Sr25 in a 7A/7E translocation into tetraploid wheat has been associated in preliminary experiments to a significant yield penalty (J. Dubcovsky personal communication).
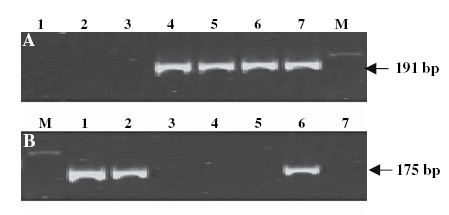
The PSY-E1 gene can be used as an additional closely linked marker to Sr25. A combination of two gene specific markers for PSY-E1 and PSY-D1 can be used to generate a codominant marker (4).
Note: you can find more details on the use of these markers for yellow pigment breeding here.
Primers sequences:
BF145935
BF145935-F 5'- CTT CAC CTC CAA GGA GTT CCA C -3'
BF145935-R 5'- GCG TAC CTG ATC ACC ACC TTG AAG G -3'
Gb
Gb-F 5'- CAT CCT TGG GGA CCT C -3'
Gb-R 5'- CCA GCT CGC ATA CAT CCA -3'
PSY-D1 specific
PSY1_DF2 5'- TTG CAG TGC AAT GGT TTT CCA -3'
PSY1_R3 5'- GAC TCC TTT GAC GAT GTC TTC -3'
PSY-E1 specific (*)
PSY1_EF2 5'- CTA CGT TGC GGG CAC CGT T -3'
PSY1_ER4 5'- AGA GAA AAC CAT TGC ATC TGT A -3'
* PSY_EF2 sequence in Ref. 4 is wrong. The correct sequence is shown here (W. Zhang, personal communication)
PCR conditions for BF145935:
- Denaturing step: 94°C, 4 min
- Amplification step (35 cycles):
- 94°C, 45 sec
- 50°C, 30 sec
- 72°C, 45 sec
- Extension step: 72°C, 7 min
PCR conditions for Gb:
- Denaturing step: 94°C, 4 min
- Amplification step (35 cycles):
- 94°C, 45 sec
- 50°C, 30 sec
- 72°C, 45 sec
- Extension step: 72°C, 7 min
PCR conditions for PSY alleles:
- Denaturing step: 94°C, 4 min
- Touchdown step (10 cycles):
- 94°C, 20 sec
- 63°C, 20 sec (decrease 0.5°C every step)
- 72°C, 1 min 20 sec
- Amplification step (35 cycles):
- 94°C, 20 sec
- 58°C, 20 sec
- 72°C, 1 min 20 sec
- Extension step: 72°C, 7 min
- Standby at 15°C
PCR reaction mix for PSY alleles amplification
- 2 µl of genomic DNA (50 ng/µl)
- 2.0 µl 10X Taq polymerase buffer
- 0.5 µl Forward primer (20 pmole)
- 0.5 µl Reverse primer (20 pmole)
- 2.0 µl dNTPs (2 mM each)
- 2.0 µl MgCl2 (15mM)
- 0.2 µl Taq polymerase (1 unit)
- 10.8 µl deionized water
Total: 20 µl
Expected products:
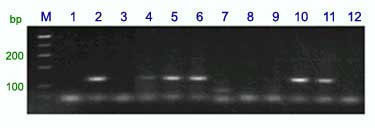
Marker BF145935 produced the fragment sizes of 198 and 180 bp in Sr25 lines and 202 and 180 bp in wheat lines without Sr25 (a detail of the amplification products is shown on Fig. 1). The dominant marker Gb amplified a 130 bp fragment only in the Sr25-positive lines and no PCR product was obtained in wheat lines that lack Sr25 (7) (Fig. 2). The combined codominant marker system for PSY-E1 is composed of two dominant markers, one for PSY-E1, linked to Sr25, that amplifies a 191-bp band, and a second dominant marker specific for PSY-D1, that amplifies a PCR product of 175-bp (Fig. 3).
References
1. Compensation indexes of radiation-induced wheat Agropyron-elongatum translocations conferring resistance to leaf rust and stem rust. Friebe B, Jiang J, Knott DR, Gill BS. In: Crop Science, 1994, 34:400-404.
2. Molecular characterization of durum and common wheat recombinant lines carrying leaf rust resistance (Lr19) and yellow pigment (Y) genes from Lophopyrum ponticum. Zhang W, Lukaszewski AJ, Kolmer J, Soria MA, Goyal S, Dubcovsky J. In:Theoretical and Applied Genetics, 2005, 111:573-582. DOI:10.1007/s00122-005-2048-y.
3. Mutation of a gene for yellow pigment linked to Lr19 in wheat. Knott DR. In: Canadian Journal of Genetics and Cytology, 1980, 22:651–654
4. Association between allelic variation at the Phytoene synthase 1 gene and yellow pigment content in the wheat grain.Zhang W, Dubcovsky J. In: Theoretical and Applied Genetics, 2008, 116:635-645. DOI:10.1007/s00122-007-0697-8.
5. Breeding triple rust resistant wheat cultivars for Australia using conventional and marker-assisted selection technologies. Bariana HS, Brown GN, Bansal UK, Miah H, Standen GE, Lu M. In: Australian Journal of Agricultural Research, 2007, 58:576-587. DOI:10.1071/AR07124.
6. Trigenomic chromosomes by recombination of Thinopyrum intermedium and Th. ponticum translocations in wheat.Ayala-Navarrete L, Bariana HS, Singh RP, Gibson JM, Mechanicos AA, Larkin PJ. In: Theoretical and Applied Genetics, 2007, 116:63-75. DOI:10.1007/s00122-007-0647-5.
7. Haplotype diversity of stem rust resistance loci in uncharacterized wheat lines.Yu L-X, S. Liu, J. A. Anderson, R. P Singh, Y. Jin, J. Dubcovsky, G. Brown-Guidera, S. Bhavani, A. Morgounov, Z. He, J. Huerta-Espino, M. E. Sorrells. In: Molecular Breeding, 2010, in press.
8. Diagnostic and co-dominant PCR markers for wheat stem rust resistance genes Sr25 and Sr26. Liu S, Yu L-X, Singh RP, Jin Y, Sorrells ME, Anderson JA. In: Theoretical and Applied Genetics, 2010, 120:691–697. DOI:10.1007/s00122-009-1186-z.
