Contributed by I.Z. Lowe and M.A. Soria
Markers for Sr26
Originally introgressed into the distal region of the long arm of hexaploid wheat chromosome 6A via an alien segment from Agropyron elongatum (syn. Thinopyrum ponticum) (1), Sr26 is one the few known major resistance genes effective against the Sr31-virulent race Ug99 (TTKSK) and its Sr24-virulent derivative (TTKST). Until now, Sr26 has been utilized as a source of resistance only in Australia, where Dr. Knott generously made his original translocation line available and where the cultivar Eagle was released in 1971. Evidence of its subsequent limited deployment is reflected in the fact that not a single instance of the resistance gene was found in a recent screen of 170 diverse lines from around the world (2). The limited historical use of the gene is likely related to a reported 9% yield penalty associated with the original 6AS.6AL-6Ae#1Lsegment (3). Recognizing the significance of this obstacle to the wider deployment of this valuable source of resistance, new translocation lines with reduced Ag. elongatum segments were developed that have been observed not to exhibit the reported yield penalty of the original Sr26 lines (4).
The effectiveness of this gene against the TTKS family of races, its low frequency among modern cultivars, and the availability of donor lines with reduced alien segments makes Sr26 ideal for use by breeding programs pursuing a strategy of marker-assisted gene stacking in anticipation of or response to the presence of Ug99 and its derivatives.
Primers sequences:
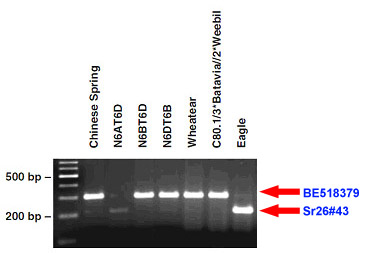
A combination (i.e. multiplexing) of two dominant PCR markers in repulsion phase provides a diagnostic co-dominant marker for Sr26: Sr26#43, a dominant marker for the presence of Sr26 (5); and BE518379, dominant for the absence of Sr26 (2)
Sr26#43
Sr26#43-F 5'- AAT CGT CCA CAT TGG CTT CT -3'
Sr26#43-R 5'- CGC AAC AAA ATC ATG CAC TA -3'
BE518379
BE518379-F 5'- AGC CGC GAA ATC TAC TTT GA -3'
BE518379-R 5'- TTA AAC GGA CAG AGC ACA CG -3'
PCR conditions:
- Denaturing step: 94°C, 3 min
- Amplification step (35 cycles):
- 94°C, 60 sec
- 60°C, 60 sec
- 72°C, 120 sec
- Extension step: 72°C, 10 min
Note: The multiplexed PCR reaction should contain 400 nM of each of the four primers, not the standard 100 nM.
Expected products:
Sr26#43 amplifies a fragment of 207 bp when Sr26 is present, and BE518379 yields a fragment of 303 bp in the absence of Sr26.
Distribution of Donor Germplasm
Four Sr26 donor lines with reduced Ag. elongatum (i.e. 6Ae#1) segments (WA1, WA5, WA6, and WA9) were developed by Dundas et al. (4). To obtain seed of these recombinant donor lines for your breeding program, please contact:
In the United States and Canada:
Dr. Scott D. Haley
Colorado State University
scott.haley@colostate.edu
Outside the United States and Canada:
Dr. Ian S. Dundas
The University of Adelaide
ian.dundas@adelaide.edu.au
Disclaimer
Alien translocations contain many genes, not simply the gene under investigation (in this case, Sr26). Be advised that reports of the effects of such segments on non-target traits (e.g. yield, quality, maturity, etc.) are specific to the genotypes and environments under which they were studied and may not necessarily be true for all genotypes and production environments.
References
1. The inheritance of rust resistance. VI. The transfer of stem rust resistance from Agropyron elongatum to common wheat. Knott DR. Canadian Journal of Plant Science, 1961, 41:109-123.
2. Diagnostic and co-dominant PCR markers for wheat stem rust resistance genes Sr25 and Sr26. Liu S, Yu L, Singh RP, Jin Y, Sorrells ME, Anderson JA. Theoretical and Applied Genetics. Published online 31 October, 2009. DOI: 10.1007/s00122-009-1186-z.
3. Grain yields of near-isogenic lines with added genes for stem rust resistance. The TT, Latter BDH, McIntosh RA, Ellison FW, Brennan PS, Fisher J, Hollamby GJ, Rathjen AJ, Wilson RE. In "Proceedings of the Seventh International Wheat Genetics Symposium", 13-19 July 1988, pp 901-906. Eds. Miller TE, Koebner RMD (Cambridge University: Cambridge, UK)
4. New sources of rust resistance from alien species: meliorating linked defects and discovery. Dundas IS, Anugrahwati DR, Verlin DC, Park RF, Bariana HS, Mago R, Islam AKMR. Australian Journal of Agricultural Research, 2007, 58: 545–549. DOI: 10.1071/AR07056
5. Development of PCR markers for the selection of wheat stem rust resistance genes Sr24 and Sr26 in diverse wheat germplasm. Mago R, Bariana HS, Dundas IS, Spielmeyer W, Lawrence GJ, Pryor AJ, Ellis JG. Theoretical and Applied Genetics, 2005, 111: 496–504. DOI: 10.1007/s00122-005-2039-z.
