Contributed by Laura Pfluger (pfluger.laura@inta.gob.ar)
The quality of wheat flour for bread making depends on the viscoelastic properties of the dough, which are influenced by the quantity and quality of the gluten-forming storage proteins of the endosperm.
These proteins consist of two classes, monomeric gliadins and polymeric glutenins, a classification based on the disulfide-bonding behavior of the individual proteins. The gliadins are monomeric proteins that either lack cysteine residues (omega gliadins) or have only intra-chain disulfide bonds. Glutenin subunits bind to each other forming polymers linked by disulphide bonds.
After reduction of disulfide bonds, glutenin subunits can be divided in two main groups: high molecular weight glutenin subunits (HMW-GS) and low molecular weight glutenin subunits (LMW-GS), based on the relatives mobilities in SDS-poyacrylamide gel electrophoresis (SDS-PAGE). Glutenin composition, especially that of the HMW-GS fraction, determines gluten strength and elasticity (1).
High Molecular Glutenins Loci
Three different loci, located on the long arms of group 1 chromosomes, code for the HMW-GS Glu-A1, Glu-B1 and Glu-D1. (2), whereas LMW-GS are coded by gene families located on the short arms of the same chromosomes.
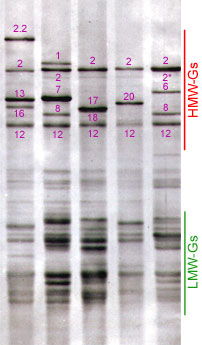
The HMW-Gs can be classified according to their electrophoretic mobility, their structure and composition. Molecular analyses have shown that each Glu-1 locus contains two genes, one encoding a higher molecular weight x-type subunit, the other a lower molecular weight y-type subunit. Absence of subunits in some cases has been proved to be due to gene silencing. Most of the bread wheat cultivars possess from three to five active HMW-Gs. Usually, the Glu-D1 locus encodes both types, the Glu-B1 locus encoded both types or one x-type subunit, and the Glu-A1 locus can have one or none active subunits (3)
HMW-GS and Gluten Strength
In bread wheat, the first studies were concentrated on HMW-GS because they have a significant impact on dough cohesion and because they are easily identified by electrophoresis.
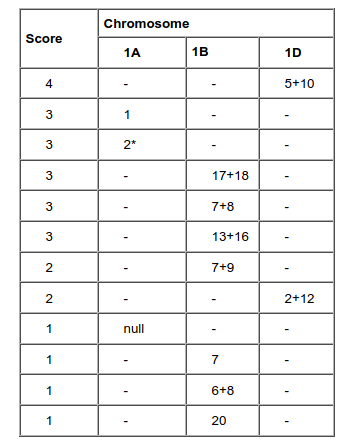
Payne et al. (4) discovered a correlation between the presence of certain HMW-GS and gluten strength, measured by the SDS-sedimentation volume test. On this basis, they designed a numeric scale to evaluate bread-making quality as a function of the described subunits (4, 5). Assuming the effect of the alleles to be additive, the bread making quality was predicted by adding the scores of the alleles present in the particular line. It was established that the allelic variation at the Glu-D1 locus have a greater influence on bread-making quality than the variation at the others Glu-1 loci.
Different reports indicate that subunit combination 5+10 for locus Glu-D1 (Glu-D1 5+10) renders a stronger dough than Glu-D1 2+12. It has been suggested that the superior effect of the 5+10 pair of subunits compared to 2+12 is largely due to the presence of an extra cysteine residue in the Dx-5 subunit compared to the Dx-2 subunit, which would promote the formation of polymers with larger size distribution. Similarly, it has been postulated that differences in the number of cysteine residues are responsible for the larger amount of large-sized polymers associated with the 17+18 pair, when compared to the 20x + 20y pair (both are present at the Glu-B1 locus). The most striking difference between these two alleles is the lack of two cysteine residues in subunit 20 (6).
The variation on bread-making quality among different varieties cannot be explained only by the variation in HMW-GS composition. The LMW-GS (and in a smaller proportion the gliadins) and their interactions with the HMW-GS also play an important role in the determination of gluten strength and breadmaking quality.
|
|
The 1BL/1RS rye translocation
Another factor that influence quality is the presence of certain wheat-rye translocations. Translocation of the short arm of rye chromosome 1R (1RS) confers to wheat resistance to a number of diseases and pathogens, and may enhance grain yield. 1RS has been transferred to wheat in the form of 1AL/1RS, 1BL/1RS and 1DL/1RS wheat-rye translocations. One of the most widely used translocation for breeding is the 1BL/1RS translocation.
The 1BL/1RS translocation has been widely used in bread wheat breeding programs, mainly because of the presence of genes for resistance to powdery mildew, stripe rust, leaf rust and stem rust on 1RS. It is estimated that several hundred cultivars with this translocation have been released worldwide (7). While the disease resistance may now be of little value, the translocation is still useful because it increases yield in some environments (8) or at least influence grain size (9). The nature of the yield advantage associated with the translocation of 1RS in wheat is not clear but may be due to the larger root biomass of the lines carrying the 1RS/1BL translocation (10, 11). Unfortunately, its presence is associated with a serious quality defect, including low sedimentation volume, dough stickiness and reduced dough strength, which disqualifies it from breeding programs developing high quality wheats (9, 12).
The short arms of the group-1 chromosomes of wheat carry several loci encoding LMW-GS, whereas the short arm of rye chromosome 1 carries the secalin Sec-1 locus that code for proteins that do not belong to the gluten fraction. The negative effect of the 1RS/1BL translocation on bread-making quality is probably related to the negative effect of the secalins and to the reduction of the number of the gluten-encoding loci and the resulting lower amount of gluten. In fact, the 1AL/1RS translocation (found in the wheat cv. Amigo), also diminishes quality, but the effect is not as severe as that observed in the varieties carrying the 1BL/1RS translocation. The loss of gluten proteins is not as great in wheats carrying 1RS/1AL translocation as in those carrying the 1RS/1BL translocation (13).
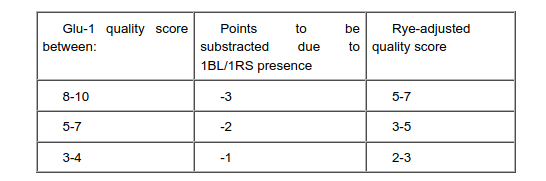
Taking into account the detrimental effects of the 1BL/1RS on bread making quality, a rye-adjusted Glu-1 quality score was calculated by Payne and co-workers. The correction was applied assuming that the decrease in quality due to this translocation would be proportionately greater in genotypes having better intrinsic quality (5).
Molecular markers for glutenins
co-dominant PCR marker to amplify the Bx7 Over expressor (Bx7OE) region
SDS-PAGE has been used extensively to identify HMW-Gs alleles and to study their effects on bread-making quality. But a closer examination of HMW-Gs has revealed that sometimes different subunits can appear to have same relative mobility in SDS-PAGE, resulting in the incorrect identification of some HMW-Gs alleles that are functionally distinct (14, 15). Recently, combined chromatographic (RP-HPLC) and PCR analyses have allowed to further differentiate bread wheat varieties apparently possessing the same allelic pair at the Glu-B1 locus (7+8, Glu-B1b allele) into four different alleles with contrasting effects on quality: Glu-B1b (Bx7+By8), Glu-B1al (Bx7OE+By 8*), Glu-B1ak (Bx7*+By8*) and Glu-B1u (Bx7*+By 8). Whereas 7 and 7* subunits could be differentiated by some special protocols by SDS-PAGE, 7 and 7OE, and 8 and 8* have identical mobilities. The large positive effect on bread-making quality of the Glu-B1al allele is notorious. The presence of this allele results in a significant increase in the expression of the Glu-B1x subunit. Cultivars with over-expression of Bx7 give mean SDS-sedimentation values superior to those cultivars not exhibiting over expression. This over-expression results in an increase of gluten strength and is considered the reason for the positive relationship between Glu-B1 al allele and bread-making quality (16-18).
Marchylo et al (19) reported variation in the staining intensity of subunit Bx7 and D’Ovidio et al (20) showed that the high quantity of expressed protein of subunit Bx7 in cultivar Red River 68 was probably due to the presence of a duplication of its encoding gene. They speculated that the favourable mixing properties of this cultivar may result in part from the quantitative increase in expressed protein that accompanied duplication of Bx gene. Gianibelli et al (14) using HPLC also found different relative amounts of subunit Bx7, indicating the over expression of this band in some cultivars. Nowadays, the cause of over-expression in most cultivars has not be determined, it may be due to gene duplication, but some other mechanism, such as more efficient transcription can be involved.
A co-dominant PCR marker has been developed for the Bx7 Over expressor (Bx7OE) region of the Bx7 gene (21), which can distinguish Bx7 from lines with Bx7OE. Lines with Bx7OE showed a 43bp insertion in that region. The Bx7OE marker is not a perfect marker for the Glu-B1al allele, since this insertion is present in other lines with allelic variants Glu-B1 ak (7*+8*) and Glu-B1a (7+By null). However, these variants can be distinguished by SDS-PAGE. A combination of SDS-PAGE and PCR amplification can be used to screen between genotypes with Glu-B1b and Glu-B1al.
A co-dominant PCR marker has been designed by Butow et al to amplify the Bx7OE region 750 bp upstream of the coding region. All of the varieties possessing Bx7OE produced a fragment of 563 bp relative to that in the gene of lines without the Glu-B1 al allele (520 bp).
Wheat DNA extraction
Genomic DNA can be extracted from 10-15 day old hypocotyls of germinating seeds using two different methods (22, 23).
Primers to amplify the Bx7OE region:
Forward 5'- CCT CAG CAT GCA AAC ATG CAG C -3'
Reverse 5'- CTG AAA CCT TTG GCC AGT CAT GTC -3'
PCR mix
- 1.5 U Taq DNA polymerase
- 10 mM Tris-HCl
- 50 mM KCl
- 1.5 mM MgCl2
- 0.2 mM dNTP
- 1 µM forward primer
- 1 µM reverse primer
- 100 ng template DNA
- final volume: 25 µl
PCR Program
- Denaturing step: 95ºC, 5 min
- Amplification step (38 cycles):
- 95ºC, 30 sec
- 58ºC, 30 sec
- 72ºC, 1 min
- Extension step: 72ºC, 5.25 min
- Hold at 4ºC
KASP marker for Glu-A1
This KASP marker separates the 1 and 2* alleles from the null allele of the Glu-A1 loci present on chromosome 1A.
|
SNP ID |
wMAS000013 |
|
Gene |
Glu-A1 |
|
Evidence |
causal |
|
Primer Allele FAM |
AAGTGTAACTTCTCCGCAACA |
|
Primer Allele VIC |
AAGTGTAACTTCTCCGCAACG |
|
Primer Common |
GGCCTGGATAGTATGAAACC |
|
FAM allele |
A |
|
VIC allele |
G |
|
FAM phenotype |
2*,1 |
|
VIC phenotype |
null |
This KASP marker was developed based on a previous PCR protocol detailed in (14).
KASP marker for Glu-D1
This KASP marker separates the 5+10 allele from the 2+12 and other alleles present on the Glu-D1 loci of chromosome 1D.
|
SNP ID |
wMAS000014 |
|
Gene |
Glu-D1 |
|
Evidence |
linked |
|
Primer Allele FAM |
ATAGTATGAAACCTGCTGCGGAG |
|
Primer Allele VIC |
ATAGTATGAAACCTGCTGCGGAC |
|
Primer Common |
TACTAAAAAGGTATTACCCAAGTGTAACTT |
|
FAM allele |
C |
|
VIC allele |
G |
|
FAM phenotype |
2+12 or others |
|
VIC phenotype |
5+10 |
This KASP marker was developed based on a previous PCR protocol detailed in Ref. 15.
References
1. Functional properties of wheat glutenin. Weegels PL, Hamer RJ, Schofield ID. In: Journal of Cereal Science, 1996, 23:1-18. DOI:10.1006/jcrs.1996.0001
2. Genetics of wheat storage proteins and the effect of allelic variation on bread-making quality. Payne PI. In: Annual Reviews of Plant Physiology, 1987, 38: 141-153. DOI;10.1146/annurev.pp.38.060187.001041
3. Structural differences in allelic glutenin subunits of high and low Mr and their relationships with flour technological properties. Lafiandra D, Masci S, D’Ovidio R, Turchetta T, Margiotta B, Mac Ritchie F. In: Wheat structure: biochemistry and functionality. J.P. Schofield (ed.), 1995, The Royal Society of Chemistry. Special publication nº 212. pp. 117-127.
4. The relationship between HMW glutenin subunit composition and the bread-making quality of British-grown wheat varieties. Payne PI, Nigtingale MA, Krattiger AF, Holt LM.In: Journal of the Science of Food and Agriculture, 1987, 40:51-65. DOI:10.1002/jsfa.2740400108
5. The HMW glutenin subunit and gliadin compositions of German-Grown wheat varieties and their relationship with bread-making quality. Rogers WJ, Payne PI, Harinder K. In: Plant Breeding, 1989, 103:89-100. DOI:10.1111/j.1439-0523.1989.tb00356.x
6. Purification and characterisation of High Mr Glutenin subunit 20 and its Linked y-type subunit from durum wheat. Buonocore F, Caporale C, Lafiandra D. In: Journal of Cereal Science, 1996, 23:195-201. DOI:10.1006/jcrs.1996.0020
7. The challenge: one billion tons of wheat by 2020. Braun HJ, Payne TS, Morgunov AI, van Ginkel M, Rajaram S. In: Proc. 9th Int. Wheat Genet. Symp. A.E. Slinkard (ed.), 1998, Saskatoon, Canada.
8. Agronomic performance of chromosomes 1B and T1BL.1RS near-isolines in the spring bread wheat Seri M 82. Villareal RL, Banuelos O, Mujeeb-Kazi A, Rajaram S. In: Euphytica, 1998, 103: 195-202. DOI:10.1023/A:1018392002909
9. Bread making quality and yield performance of 1BL/1RS wheat isogenic lines. Bullrich L, Tranquilli G, Pfluger L, Suárez E, Barneix A. In: Plant Breeding, 1998, 117:119-122. DOI:10.1111/j.1439-0523.1998.tb01463.x
10. Root biomass, water-use efficiency and performance of wheat rye translocations of chromosomes 1 and 2 in spring bread wheat 'Pavon'. Ehdaie B, Whitkus RW, Waines JG. In: Crop Science, 2003, 43:710-717. DOI:10.2135/cropsci2003.7100
11. 1RS translocation increases root biomass in Veery-type wheat isogenic lines and associates with grain yield . Ehdaie B, Waines JG. In: p 693-695. Proc. 10th. Intern. Wheat Genet. Symp. Paestum, Rome, Italy. N.E. Pogna ed.
12. Relationship between wheat high molecular weight glutenin subunit composition, 1RS translocations and sodium dodecyl sulfate sedimentation volume. Pflüger L, Suárez E, Lafiandra D. In: Journal of Genetics and Breeding, 1998, 52:271-279
13. Uneasy unions: quality effects of rye chromatin transfers to wheat. Graybosch RA. In: Journal of Cereal Science, 2001, 33:3-16. DOI:10.1006/jcrs.2000.0336
14. New DNA markers for high molecular weight glutenin subunits in wheat. Liu S. Chao S, Anderson JA. Theoretical and Applied Genetics, 2008, 118:177-183. DOI:10.1007/s00122-008-0886-0
